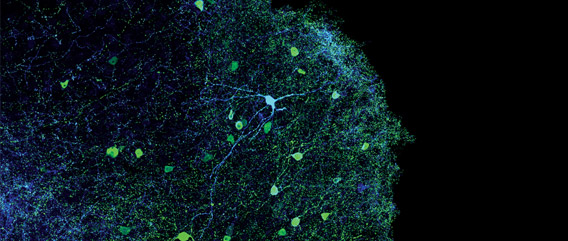
The Optogenetics Revolution
A mouse enters an environment that it previously learned to associate with a mild footshock. It freezes, crouching in anticipation of an electric shock. But then, a fiber optic filament beams light deep into its brain, shutting off the neurons that encoded this fear memory. The mouse begins its normal investigation of the environment, all traces of fear erased with a flash of light. This is the power of optogenetics: the ability to switch neurons on or off with light and to map, with exquisite precision, the neural circuitry of the brain.
Since the advent of modern neuroscience, scientists have sought to understand how the billions of neurons in our brains act separately but in concert to create what we experience as learning, thinking, memory, and behavior. However, the endeavor to understand the intricacies of the functioning brain has long been limited by the difficulty of observing and manipulating neurons as they work together in a live, behaving animal.
“Understanding how the 100 billion nerve cells in our brain function to generate sensation, movement, memory, and thought is one of the most fascinating and challenging problems in science,” says Steven Siegelbaum, PhD,
chair of neuroscience at P&S.
‘I want to stimulate cells using light to see if I can help them stay alive and improve memory. If you can acutely fix a memory impairment using this paradigm, can you chronically stimulate them with light to keep them alive and active?’
Until recently, even the most precise tools for investigating living, functioning brains, including fMRI, could only yield images of neuronal activity on a large scale—what René Hen, PhD, professor of neuroscience and of psychiatry, calls “blobs of brain consisting of many cell types.” At the other end of the spectrum, researchers interested in the function of a single type of neuron were limited to recording electrical signals from a candidate cell with a microelectrode and then painstakingly hunting down its synaptic partner with a second electrode. Only after the experiment was over would microscopic imaging of fixed brain slices reveal which neuron type had been probed.
Those who wished to experimentally manipulate the brain were similarly stymied, limited to crude and irreversible techniques such as removing tissue and observing the effects or using genetically engineered mouse models. Selective and reversible activation or deactivation of specific populations of neurons simply was not possible.
Today, the field of optogenetics has changed all that. Optogenetics allows researchers to insert light-sensitive molecules, called opsins, into specific populations of neurons. Then, with light beamed in through fiber optic strands, researchers can turn those neurons on or off with millisecond precision.
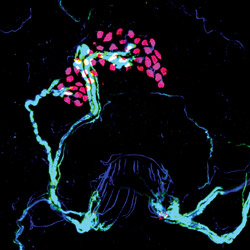 Optogenetics is not just for brain scientists. Sensory neurobiologist Ellen Lumpkin used the technique in skin cells for the first time, revealing new insights about the way we are able to sense fine details and textures with our fingertips. She recently discovered that skin cells called Merkel cells (labeled pink) work together with neurons (light blue) to transmit information about touched objects to the brain. Learn more at bit.ly/merkelcells
Optogenetics is not just for brain scientists. Sensory neurobiologist Ellen Lumpkin used the technique in skin cells for the first time, revealing new insights about the way we are able to sense fine details and textures with our fingertips. She recently discovered that skin cells called Merkel cells (labeled pink) work together with neurons (light blue) to transmit information about touched objects to the brain. Learn more at bit.ly/merkelcells“Optogenetics has given us a whole new way of controlling different types of neurons,” says Dr. Siegelbaum. “Neuroscience is now at the point where advances in mouse genetics, imaging, and the development of molecular tools such as optogenetics have converged to give us an unprecedented chance to gain insight into how the brain functions and how alterations in brain function underlie neurological and psychiatric disease.”
Lowly Beginnings
The unlikely stars of optogenetics are light-sensitive proteins from lowly archaebacteria and single-celled algae—pond scum. Decades before a practical application was developed, scientists had been probing the secrets of opsins, proteins found in these organisms that span the cell membrane and respond to light by either opening or closing ion channels—which, conveniently, can trigger the neural code for “on” or “off.”
In 2005, Karl Deisseroth, Edward Boyden, and Fen Zhang at Stanford published an article demonstrating that the gene for an opsin called channelrhodopsin could be inserted into selected neurons and used to switch those neurons on with a pulse of blue light. Soon, halorhodopsin was found to exert the opposite effect, turning selected neurons off with a pulse of yellow-green light. The field of optogenetics was born.
Since those early days, many new and improved optogenetics molecules have joined the list. “Optogenetics has led to a new level at which we can probe and understand how the brain works,” says Dr. Siegelbaum. “It is the perfect tool in every way for what many of us at Columbia are trying to do in terms of understanding how neural circuits control behavior.”
Understanding the Physiology of Memory
One of the most basic functions of the brain is processing and storing information. Memory is the foundation of our consciousness. It informs not just our remembrance of the past, but also our expectations of the future, our hopes, our fears. Ultimately, memory is the basis on which we form our understanding of the world.
The hippocampus—a seahorse-shaped region in the brain—is known to be associated with learning and memory, but precisely how it stores and retrieves information is not fully understood. Much of what we know about the hippocampus predates the use of optogenetics and has relied on painstakingly laborious research techniques. Optogenetics has given researchers interested in understanding the formation of memory a powerful new way of understanding the most basic questions about how the brain works.
Like the rest of the brain, the hippocampus is comprised of many different types of neurons. More than 80 percent are excitatory neurons, which tend to activate the other neurons in their networks. But while the number of inhibitory neurons, or interneurons, is relatively small, they fall into many different categories, each different type modulating the effects of excitatory neurons in a specific manner. “If you want to understand how the hippocampus processes episodic information and generates memory, you must understand how these different types of neurons interact while a memory is being formed,” says Attila Losonczy, MD, PhD, assistant professor of neuroscience.
Using optogenetics in combination with an advanced imaging technique called two-photon microscopy, Dr. Losonczy recently looked at how interneurons influence the formation of fear memories. When neurons are active, they accumulate calcium. In mice that are genetically engineered to express calcium-sensitive fluorescing proteins, those activated neurons produce a tiny flash of light. “Two-photon imaging allows us to track the activity of individual neurons during the course of learning—and we can perform this imaging for days to weeks because the calcium-sensitive fluorescing sensor is permanently expressed in the neuron,” he says.
This technique has enabled Dr. Losonczy to identify which of the myriad interneurons in the hippocampal circuit are activated in contextual fear learning—a well-validated technique for investigating memory in mouse models.
His findings, reported in the Feb. 20 issue of the journal Science, show that not all types of interneurons in the hippocampus are equal. He found that interneurons that innervate the dendrite, or input channel, of excitatory neurons were especially important in generating memory. “When we silenced these neurons, we found the animal was not able to learn and remember the context where a fearful event occurred. Moreover, the precise temporal resolution of optogenetics allowed us to reveal that these interneurons play their key role during the presence of the fearful stimulus,” says Dr. Losonczy. “On the other hand, when we manipulated the activity of the interneurons that innervate the cell body, there was no deficit in contextual fear learning.”
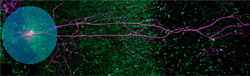 New hippocampal microcircuits involved in long-term synaptic plasticity, the cellular basis of episodic memory formation (the memory of people, places, and events), were discovered using optogenetics. By shining light (blue dot) in hippocampal slices, Jayeeta Basu, PhD, an associate research scientist in Dr. Steven Siegelbaum’s lab, turned on local inhibitory neurons, revealing their inhibitory effect on CA1 pyramidal neurons (pink, the principal hippocampal output neurons). Adapted from Basu et al., Neuron 2013.
New hippocampal microcircuits involved in long-term synaptic plasticity, the cellular basis of episodic memory formation (the memory of people, places, and events), were discovered using optogenetics. By shining light (blue dot) in hippocampal slices, Jayeeta Basu, PhD, an associate research scientist in Dr. Steven Siegelbaum’s lab, turned on local inhibitory neurons, revealing their inhibitory effect on CA1 pyramidal neurons (pink, the principal hippocampal output neurons). Adapted from Basu et al., Neuron 2013.The findings were the first confirmation of what had previously only been speculated. “Prior to using optogenetics, we did not have tools in our hands to look so specifically at the activity of those interneurons during learning with such precise temporal resolution,” says Dr. Losonczy. “Now is a very exciting time because we are developing the tools to be able to answer questions people have been trying to answer since the advent of neuroscience and answer them in a more precise, specific way. This is a huge step forward, but we are just at the beginning of this journey.”
The Physiology of Forgetting
Determining how memories are formed is a crucial step in understanding how the brain works. But where are those memories stored in the short and long term? What is happening in the brain when memories fade? And can the cumulative failures of memory that creep in with age and disease be stopped or reversed?
Christine Ann Denny, PhD, assistant professor of psychiatry and recipient of the NIH’s prestigious Early Independence Award, uses the power of optogenetics to gain a deeper understanding of how and why memory fails. Dr. Denny focuses on how the brain encodes and retrieves short- and long-term memories and why memory breaks down in memory-related disorders such as depression and Alzheimer’s disease. She focuses on two subregions of the hippocampus known to be directly involved in the formation of new memories, the dentate gyrus and CA3.
“I want to characterize memory traces—what happens when an animal learns as opposed to when it doesn’t learn? What happens when it learns but cannot retrieve the memory? Where are the impairments in aging or in Alzheimer’s disease?”
During her graduate work at Columbia in the laboratory of Dr. Hen, Dr. Denny developed a genetic mouse line that enabled her to permanently express a fluorescent label in the neurons that were activated during a particular memory. As she trained mice to fear a particular context, she was able to tag the cells that participated in learning with one color. Later, when the mice were re-exposed to the same fearful context, she was able to label the cells that were activated in memory retrieval with another color. The cells that had overlapping labels represent what she calls a memory trace.
The permanence of these labels has allowed her to look beyond the typical short-term window for studying memory traces and, therefore, she is able to see that the memory traces in the hippocampus become fainter over time, even though conditioned behaviors persist. “This indicates that the memory is not as reliant on the hippocampus for behavioral expression and has possibly been redistributed to other areas of the brain,” she says.
She hopes that discovering how short-term memories become long-term memories—and where those memory traces go when they are no longer as reliant on the hippocampus—will shed light on how aging and Alzheimer’s disease impair both kinds of memory. The work also may lead to new interventions. “Alzheimer’s disease has both short- and long-term memory loss. You can forget where you put your keys but you can also forget the names and faces of your children. I want to characterize how different memories are stored and retrieved and where the dysfunction occurs in memory-related disorders,” she says.
If these weaker memories are not being accessed properly, can the neurons that encode them be selectively stimulated using optogenetics techniques? “I want to stimulate the cells using light to see if I can help them stay alive and improve memory,” says Dr. Denny. “I want to know if you can acutely fix a memory impairment using this paradigm. If so, can you chronically stimulate them with light to keep them alive and active?”
Learning, memory, and anxiety are clearly linked but the connection has never been fully understood on a neural level
Karen Duff, PhD, professor of pathology & cell biology (in psychiatry and in the Taub Institute for Research on Alzheimer’s Disease and the Aging Brain), sponsored Dr. Denny’s NIH award to recruit new investigators with optogenetics experience to the Alzheimer's disease field. Her program explores how Alzheimer’s disease interferes with memory and how optogenetics might point to new therapeutic interventions. “We’re coming more from the translational side with the hope that stimulating the brain might help dementia patients. One issue we face is what happens in a degenerating brain cell when you try to stimulate it,” she says. “Can you stimulate a cell that is sick and still improve its function, or boost the activity of surviving cells in a degenerating brain to compensate for those lost to the disease?”
In Alzheimer’s disease, for reasons not yet fully understood, the brain accumulates beta amyloid plaques and tau tangles. “At the same time we see these plaques and tangles building up, the cells and the synapses start to degenerate,” says Dr. Duff. “Ultimately you lose connections.”
In the early stages of the disease, cells are lost in the entorhinal cortex, a key conduit between the neocortex and the hippocampus. Initially patients typically present with mild memory loss but progress to profound impairment. Dr. Duff and her colleagues specifically look at the entorhinal cortex-hippocampal circuit to try to understand the precise causes of the initial problem. “With optogenetics we can investigate dysfunction in this circuit in novel mouse models with Alzheimer’s disease pathology, looking at what happens with memory in the earliest stages of the disease,” she says. “Maybe if you can tweak that stage of the disease, you could defer the disease progressing to the stages where memory loss becomes debilitating.”
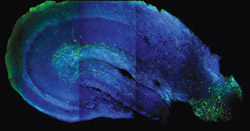 The power of optogenetics allows neuroscientists to functionally map neuronal connections across brain regions using acute brain slices. Previously, long-distance circuitry was challenging to study in slices because slicing severs connections. Optogenetic stimulation can activate even the severed projection axons at their target sites, allowing researchers to trace circuit pathways between different interconnected brain areas. Such techniques allowed Dr. Basu to uncover a “disinhibitory” long-range projection (green) from the entorhinal cortex (lower right) to the hippocampal CA1 (center) that is important for gating dendritic spikes, somatic plasticity, and contextual learning.
The power of optogenetics allows neuroscientists to functionally map neuronal connections across brain regions using acute brain slices. Previously, long-distance circuitry was challenging to study in slices because slicing severs connections. Optogenetic stimulation can activate even the severed projection axons at their target sites, allowing researchers to trace circuit pathways between different interconnected brain areas. Such techniques allowed Dr. Basu to uncover a “disinhibitory” long-range projection (green) from the entorhinal cortex (lower right) to the hippocampal CA1 (center) that is important for gating dendritic spikes, somatic plasticity, and contextual learning.Here is where Dr. Duff calls her future ambitions “a little science fiction-y.” Once the circuits involved in memory are more clearly understood, Dr. Duff wants to determine if targeted electrical stimulation of the brain could help prevent or reverse memory loss—or even retrieve receding memories in patients with Alzheimer’s disease, just as fear memories can be reactivated in mice using optogenetics. “As a short-term fix, what if you could boost the ability of a person’s brain to function whilst you train it on certain things that the patient wants to remember?” she asks. “What if you didn’t train someone to remember a fear memory as they did in the mice experiments, but the memory of a loved one’s face, for example, or key landmarks that would allow a person with dementia to better remember their local environment?”
While optogenetics is not currently a practical way to stimulate human brain cells, deep brain stimulation (DBS) or transcranial stimulation using electrode wires is being used clinically, especially for Parkinson’s disease, and is being tested for therapeutic potential in Alzheimer’s patients. “Although it is not known how these stimulation therapies work in humans, in theory they do the same thing to the cell as optogenetic stimulation. They can stimulate neural activity by depolarizing the cell,” she says. “Optogenetics allows us much finer control over what populations of cells and circuits are stimulated compared to DBS but the information gathered can then be applied to the more practical therapeutic tools. However, whether stimulation can rescue or prevent memory loss in a diseased brain is a big unknown that we will have to look at in our mouse models.”
Learning, Anxiety, and Depression
Memory malfunction comes in many forms besides loss of memory. Take anxiety, for example. On one hand, remembering and correctly identifying dangerous contexts are important adaptive traits—learning to identify risk can prevent you from blithely tripping into unsafe situations again and again. On the other hand, overgeneralized fear can lead to anxiety even in safe situations, with emotionally disabling consequences.
While learning, memory, and anxiety are clearly linked, the connection has never been fully understood on a neural level. With optogenetics, questions that were once the province of philosophy and psychology are now the domain of neuroscience. René Hen, PhD, professor of neuroscience and of psychiatry, and Mazen Kheirbek, PhD, assistant professor of clinical neurobiology in psychiatry, use the technique to dissect how learning, memory, and anxiety are linked in a portion of the hippocampus called the dentate gyrus.
“In the past, the problem has been that the neurons of the hippocampus send projections to many parts of the brain, so when you stimulate them you also get activation in all of these downstream structures,” says Dr. Hen, who is also a member of the Kavli Institute for Brain Science. “With optogenetics you can tease apart which of the many areas are important. We couldn’t do this work without the genetic trickeries and the panoply of optogenetics tools we have developed.”
Recent findings published by the Hen lab show that the link between learning and anxiety has a physical location along the dorsal-ventral axis of the dentate gyrus. When the neurons in the dorsal portion of the dentate gyrus were stimulated, learning was disrupted and exploratory activity increased. But when the same type of neurons were activated in only the most ventral part of the region, anxiety was reduced without affecting learning.
“We were able to show that cells that otherwise look the same had different functions according to their location in the dentate gyrus,” Dr. Hen says. The findings have therapeutic implications for post-traumatic stress disorder and anxiety or panic disorders, pointing to another potential use of deep brain stimulation, which is used for Parkinson’s disease and some other disorders but is not FDA-approved for treating anxiety-related disorders.
The Hen lab is also investigating the role of adult neurogenesis in depression and anxiety. “New neurons are born in parts of the adult hippocampus, and we are trying to test the role that these adult-born cells play in learning, memory, and mood. Using optogenetics allows us to control those cells in real time while the animal is behaving, to understand how they play a role in learning and memory as well as how they play a role in the anxiety state of the animal,” says Dr. Kheirbek, who was among the first researchers at P&S to use the optogenetics research technique and brought it into the Hen lab as a postdoctoral fellow.
Their initial findings suggest that the adult-born neurons play a critical role in defending against anxiety. “If you turn off those new neurons you lose the ability to distinguish between fearful and safe situations,” says Dr. Kheirbek.
The larger goal of the Hen lab is to find a way to treat psychiatric disorders using the information they glean. “In the big picture, this knowledge will allow us to develop novel antidepressants and novel anxiolytics,” says Dr. Hen, who works with pharmaceutical companies to identify therapeutic strategies that can increase the population of new neurons within the hippocampus.
Fear Factor
Although many neuroscientists look in tiny areas of the brain to focus their attention on circuits that are involved in specific neural processes, many of those processes span multiple structures of the brain that interact with one another in complex ways that scientists are only beginning to grasp. Even subtle changes in the way these regions interact with each other can lead to different behaviors.
When it comes to determining whether a situation is safe, for example, the amygdala and the prefrontal cortex are involved in a complex sort of competition, says Joshua Gordon, MD, PhD, associate professor of psychiatry. The determination of whether a situation is deemed safe is ultimately made by whether the amygdala or the prefrontal cortex leads the interaction.
To elucidate this relationship, Dr. Gordon trains mice to associate a specific tone—the fear tone—with receiving a mild shock. The same mice learn to associate a different tone—the neutral tone—with not receiving the shock. Then, using microelectrodes that can detect the electric current from a single neuron firing, Dr. Gordon applies mathematical modeling to identify the order in which neurons are activated from different regions of the brain. “After training, we found that both tones engaged synchrony between the prefrontal cortex and the amygdala,” he says, “but only the fear tone caused freezing.”
His preliminary data have shown that when the fear tone was played, the amygdala fired first, leading the prefrontal cortex. But in response to the neutral tone, the prefrontal cortex led the amygdala, “as if it was telling the amygdala, ‘It’s OK. You’re safe,’” he says. “There is this back-and-forth, and whichever region wins will determine the behavior.”
The lab is now engaged in optogenetics research that selectively silences either the amygdala or prefrontal cortex, essentially fixing the results of the contest. “It used to be that we would just make the hypotheses, but without optogenetics we would have no way of proving that, yes, there’s this competition that’s taking place,” says Dr. Gordon. “Now we can influence the competition. We can silence the prefrontal cortex and take that end of the competition away to see if the animal is more afraid than it should be.” He is currently analyzing the electrophysiological data from such an experiment in which they silenced the soothing input from the prefrontal cortex and let the amygdala take charge. “We expect that this will cause the animal to inappropriately freeze in response to the neutral tone. That has implications if we can find out how mice—and people—suppress fear responses. We can hope to use that in patients with anxiety disorders.”
A Brighter Future for Neuroscience
Optogenetics—whether described as a method, technology, application, or revolution—provides a new array of opportunities to explore the brain. “This field did not even exist when the current millennium began,” says Dr. Siegelbaum, “yet from a lowly product of nature we now have the underpinnings of a method that will provide a precise means of stimulating or inhibiting local brain regions, offering the hope of both a better understanding of neurological and psychiatric diseases, as well as, perhaps, novel treatments for these disorders.” Optogenetics is the illuminating power source of this new revolution in brain science.
- Log in to post comments